UK Fibromyalgia Treatment Trial Proves Whole Body PBM Therapy Works!
In an NHS fibromyalgia clinical treatment trial researchers from the Department of Pain Management at the Sandwell and West Birmingham Trust have used the NovoTHOR Whole Body PBM Therapy Light Bed to successfully treat the debilitating and multiple symptoms associated with Fibromyalgia.
Just like the recently published Spanish triple blind Random Controlled Trial (RCT) for Fibromyalgia (which also used the NovoTHOR whole body light bed), the NHS researchers also obtained amazing results in their clinical trial!
What happened in the trial?
- The trial lasted 8 months from January 2022 to August 2022
- 70% of the participants were Female and 30% Male (as is the norm for Fibromyalgia, there are many more Female suffers than Males)
- Ages ranged from 28 to 66 years (47 years was the average age – often peri-menopausal or menopausal women)
- The time since the diagnosis of Fibromyalgia was an average of 15.5 years (range from 4 to 31 years so all participants had long standing and deep rooted troubles)
- Treatment with the NovoTHOR Whole Body PBM Therapy Light bed consisted of three 20 minute treatments per week for 6 weeks (18 in total). This was higher than the Spanish Fibromyalgia trial where the participants had three 20 minute treatments per week for 4 weeks (12 in total)
The results were amazing!
Pain and Daily Disability Improvements
To measure how well the NovoTHOR Whole Body PBM Therapy Light Bed worked the researchers used the gold standard Revised Fibromyalgia Impact Questionnaire (FIQR). For those who do not know, the FIQR asks 21 questions about how the Fibromyalgia impacts a patients life and all the results are added together to give an overall picture of their health. It covers for example, pain levels, fatigue, depression, sleep quality, memory and ability to carry out daily activities as well as various other parameters to help measure the effect of the condition on the individual. The score varies from 100 (being the worst possible) to 0 (being nothing). In the study FIQR scores were:
- Obtained at the start
- Assessed again at 6 weeks (18 sessions) and then,
- 24 weeks later – 18 weeks after all treatment had stopped to give an indication about how long the improvements could last.
Revised Fibromyalgia Impact Questionnaire (FIQR)
- The average score at the start was 79.7/100 – profoundly disabled and limited by Fibromyalgia (100 being the worst possible score)
- At 6 weeks there was a whopping 31% improvement in the FIQR score (55.3)
- Then (not unexpectedly) the score slipped back at week 24, after the last treatment the patients still maintained a 19% improvement 4 ½ months after the last NovoTHOR treatment! FIQR score (65)
Other Amazing Results at 6 weeks
- Pain levels almost halved from 7.08 to 3.93 (44% reduction in pain)
- Manual Tender Point Survey score (MTPS) – a notable change was demonstrated across 94.4% of the participants. The total Trigger Point numbers were reduced and more pressure could be tolerated (see graph below)
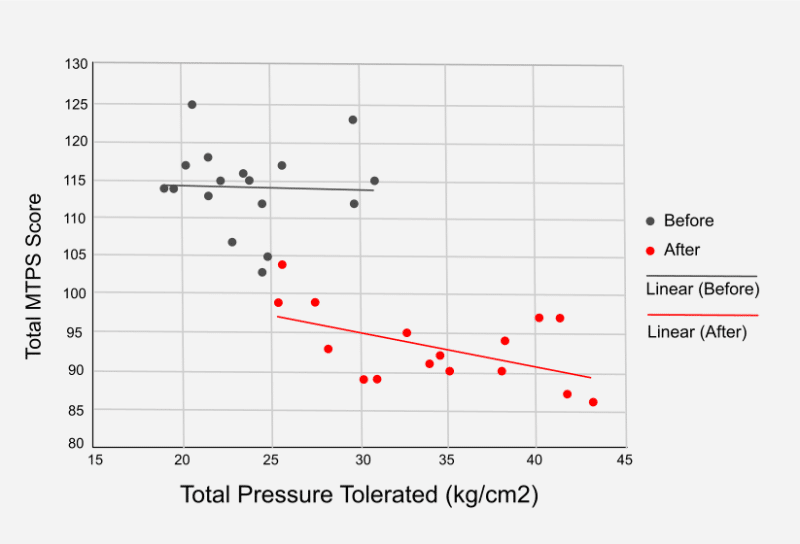
- Fatigue improved from 6.3 to 5.6 (11% improvement)
- Sleep quality improved from 17.4 to 11.5 (34 % improvement)
- ‘Patient Global’ went from 5.47 to 3.79 ( 31% improved) where patients were asked to rate the their overall quality of life, symptoms, emotions, and activity limitation related to their pain condition at the start and then again at week 24
- Anxiety and depression scores improved from 12.5 to 8.21 (34% improvement)
- Stiffness scores changed from 9.05 – 5.95 (34 % improvement)
- Dyscognition (brain fog) changed form 8.35 to 5.58 (33% improvement)
- It has already been clearly established that PBM Therapy is extremely safe and in this study the researchers did not find any adverse effects of treatment and no side effects were reported
- Medication reduction – a significant portion of the group were able to stop or markedly reduce their medications for example:
14 participants were taking 17 opioid-based medications – 64.3% of them reduced or stopped their opioid medication by Week 6 which included codeine phosphate, co-codamol 30/500, Tramadol, Oramorph® and BuTrans® – a Buprenorphine transdermal patch. Of those also taking anti depressants for pain (4) as opposed to for depression, 50% were able to stop taking them and one of these was on the maximum dose of 120mg day! At the start one patient’s dose of anticonvulsants for neuropathic pain (e.g. Gabapentin/Pregabalin) was double the maximum daily recommended dose but by Week 6, the dose was within recommended limits.
Conclusion by the Researchers
‘There appears to be a clear advantage in supporting the use of whole-body PBMT in the Fibromyalgia population’
This pioneering work represents a well-designed feasibility trial that has shown significant improvements in all Fibromyalgia domains in the short and long-term
What is a Fibromyalgia Domain?
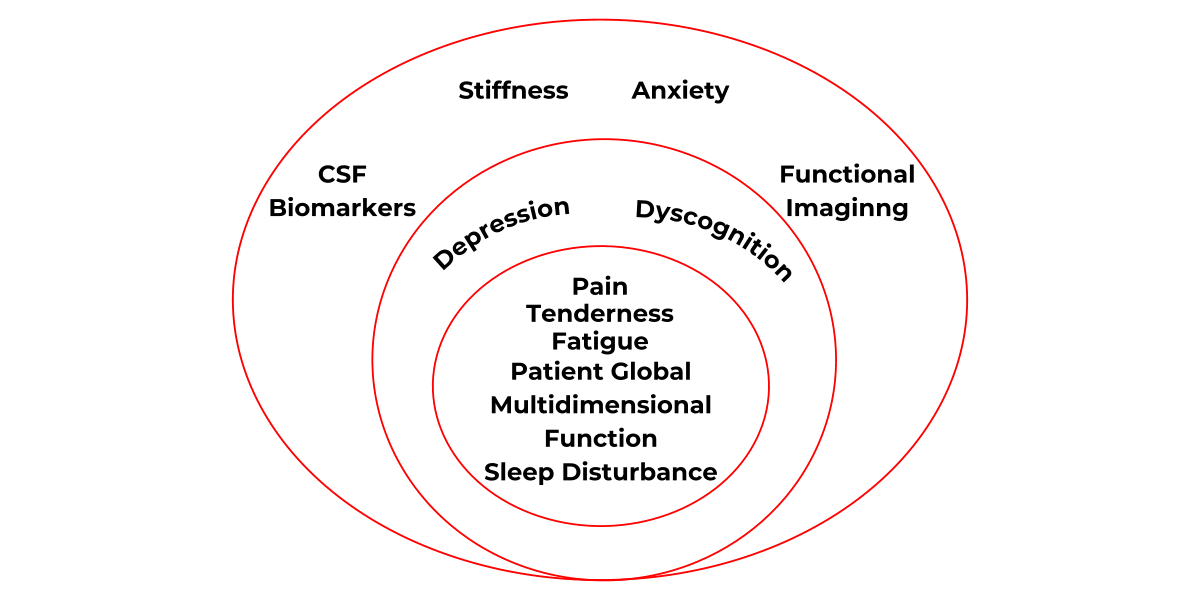
This study covered all of the above domains (symptoms) but not Functional Magnetic Resonance Imaging (FMRI) and Cerebrospinal Fluid – CSF markers (which requires a lumbar puncture to take CSF fluid for analysis). To read the actual trial just click the pdf icon below.
Authors note: It would be interesting if the study could have kept treating patients for another 18 sessions, the patients might have had double the improvements! What we have found with our Fibromyalgia patients is 18 sessions is NOT the maximum improvement or as good as you can be. If you keep going with the same frequency we have found you keep improving. Ultimately the objective is to get you to the point of maximum improvement, at which time we extend the time period between treatments to achieve a point where you don’t slip back, but equally can go as long as possible between ‘top-ups’.
Also just ‘stopping treatment’ abruptly makes no sense to me when dealing with subjects who have chronic health care needs. My clinical experience of using our NovoTHOR for patients with chronic symptoms such as Fibromyalgia, is that a few do stay well for a long time, but just like in this study many slowly slip backwards (as occurred 4.5 months after the last treatment).
So for those who do start to slip back as we go longer between appointments we do find that their improvements can be maintained with periodic ‘maintenance’ or ‘top ups’ of NovoTHOR Whole Body NovoTHOR PBM Therapy.
Is NovoTHOR Whole Body PBM Therapy Available on the NHS?
The short answer is ‘no’ and in my opinion this is unlikely to change for a considerable amount of time. Why? This was a pilot NHS trial. The next NHS step would probably be more center trials all over the UK – to prove that they can reproduce the same results. At the time of this article there are only 15 NovoTHOR whole body PBM Therapy light beds in the UK, all in private clinics who have invested huge sums of money in obtaining the equipment, creating a room for it and then managing its use. This is not a simple feat (as ours is one of those clinics) and in my opinion will probably take years before the NHS is in the same position as the existing 15 clinics, and quite possibly never.
As these 15 facilities are up and running and available now, the NHS would only need to approach us and discuss contracts…..

Our PBM Therapy Clinic has the enviable position of having one of the few whole body NovoTHOR PBM Therapy light beds in the UK. There are only a few hundred of these globally and 15 in the UK. We are easy to get to as we are in the centre of the country, close to motorways, train links and airports.
To read more just search the site and watch the videos on our PBM Therapy You Tube channel. If you have any questions, please don’t hesitate to call us on 01332 224829.

